Autophagy, the cellular recycling process vital to maintaining cellular health and preventing diseases, has become a topic of increasing interest in recent years. This fascinating process is involved in the degradation and reuse of cellular components, playing a crucial role in both health and illness. In this journey through the world of autophagy, you will explore its fundamental mechanisms, roles in health and disease, various methods of induction, and techniques for monitoring and measuring this complex process. So let’s dive in and unravel the mysteries of autophagy.
Table of Contents
Key Takeaways
Autophagy is a cellular process of breaking down and reusing components to maintain homeostasis.
It has implications in various diseases such as neurodegenerative disorders, cancer, aging, and more.
Techniques for monitoring autophagy include fluorescent markers/imaging techniques & biochemical assays.
The Fundamentals of Autophagy
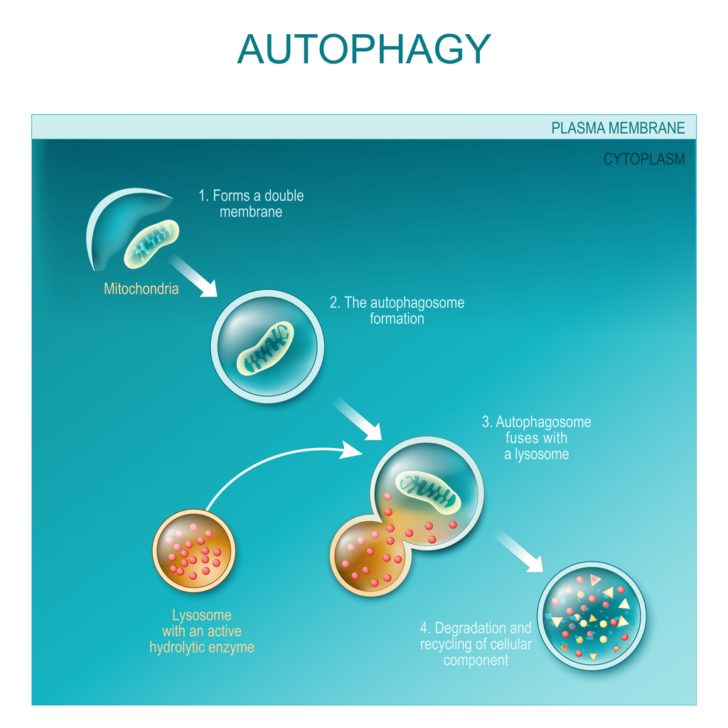
Imagine your body as a bustling city with millions of inhabitants, all working to keep the city functioning at its best. Just like a city, our body’s cells need to maintain cleanliness and order. Autophagy is the cellular equivalent of a recycling center, where the cells break down and reuse their components. This recycling process not only optimizes the performance of cells, but also keeps them in check, preventing clutter from accumulating and turning into harmful waste.
There are three primary types of autophagy that work together to maintain cellular balance: macroautophagy, microautophagy, and chaperone-mediated autophagy. Each type plays a specific role in the degradation and reuse of cellular components, ensuring the smooth functioning of our body’s cells.
We will now discuss the unique roles and mechanisms of these types in greater detail.
Macroautophagy
Macroautophagy is the most prevalent form of autophagy and is often what comes to mind when we think of cellular recycling. During this process, a portion of the cytoplasm containing damaged cell organelles, such as the endoplasmic reticulum, or unnecessary proteins, is enclosed within a double-membrane vesicle called an autophagosome. The autophagosome then fuses with a lysosome, where the captured materials are degraded and recycled.
Macroautophagy safeguards our cells by preserving cellular homeostasis and offering protection against a variety of human diseases.
For more info, visit The machinery of macroautophagy.
Microautophagy
Unlike macroautophagy, basal autophagy, which relies on the formation of autophagosomes, microautophagy directly engulfs cytoplasmic cargo at the boundary membrane by autophagic tubes. This process is more straightforward than macroautophagy but still plays a vital role in cellular recycling and maintenance.
The main difference between microautophagy and other forms of autophagy lies in the way the cargo is taken up by the lysosome, highlighting the diversity and versatility of the cellular recycling machinery.
Chaperone-Mediated Autophagy
In chaperone-mediated autophagy, specific proteins are targeted for degradation based on their amino acid sequences. This selective autophagy process allows cells to recognize and eliminate specific components, such as protein aggregates and damaged organelles, that could have harmful consequences if left unchecked. One essential player in this process is the adaptor protein p62/SQSTM1, which helps the cell recognize and target specific proteins for degradation by recognizing their amino acids.
Chaperone-mediated autophagy has been implicated in various diseases, including neurodegenerative disorders and cancer. In neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s, the removal of intracellular aggregates by autophagy is crucial for maintaining neuronal health. On the other hand, elevated p62 levels have been observed to disrupt cellular signaling and promote inflammation-associated tumorigenesis in human hepatocellular carcinoma. These findings highlight the diverse and context-dependent roles of chaperone-mediated autophagy in health and disease.
Roles of Autophagy in Health and Disease
Autophagy is a double-edged sword, playing both protective and detrimental roles in health and disease. On one hand, it is responsible for maintaining cellular homeostasis and preventing cellular dysfunction, which can contribute to aging and age-related diseases. On the other hand, dysregulation of autophagy has been implicated in various diseases, including neurodegenerative disorders and cancer, sometimes promoting the progression of the disease.
To gain a deeper understanding of autophagy’s complex roles in health and disease, we will examine its involvement in cell survival, aging, neurodegenerative diseases, and cancer.
Cell Survival and Aging
The maintenance of cellular homeostasis and prevention of cellular dysfunction, which can lead to aging and age-related diseases, hinge on autophagy. By breaking down and recycling damaged proteins and organelles, autophagy helps cells stay clean and efficient, much like a well-maintained city. In this context, autophagy can be seen as a cellular fountain of youth, keeping cells in their prime and warding off the ravages of time.
However, the relationship between autophagy and aging is not entirely straightforward. While autophagy helps maintain cellular health, its efficiency may decline with age, contributing to the accumulation of damaged proteins and organelles and the onset of age-related diseases. This highlights the delicate balance between autophagy and aging and emphasizes the importance of maintaining proper autophagy function throughout life.
Neurodegenerative Diseases
Dysregulation of autophagy has been implicated in the development and progression of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s disease. In these disorders, the buildup of damaged proteins and organelles can lead to neuronal dysfunction and cell death, emphasizing the importance of maintaining proper autophagy function.
Exploring neuronal autophagy as a potential therapeutic target for neurodegenerative diseases is a vibrant field of research. By enhancing or modulating autophagy, researchers hope to develop treatments that can slow or halt the progression of these devastating diseases. However, much remains to be learned about the precise mechanisms and roles of autophagy in neurodegeneration, as well as the optimal strategies for harnessing its potential as a therapy.
Cancer and Tumor Suppression
The relationship between autophagy and cancer is a complex and intriguing one. Autophagy can act as a tumor suppressor by eliminating damaged organelles and preventing genomic instability. However, in certain circumstances, autophagy can also promote tumor growth and survival, providing cancer cells with the energy and resources they need to thrive.
This dual nature of autophagy in cancer raises both challenges and opportunities for therapeutic intervention. On one hand, enhancing autophagy may help prevent the development of cancer by eliminating damaged cellular components and maintaining genomic stability. On the other hand, inhibiting autophagy in established tumors may deprive cancer cells of the resources they need for tumor cell survival and growth, potentially improving the effectiveness of cancer treatment.
Further research is needed to better understand the complex roles of autophagy in cancer and to develop targeted therapies that can exploit its unique properties.
Inducing Autophagy: Methods and Considerations

Given the importance of autophagy in health and disease, researchers and individuals alike are interested in ways to induce or modulate this process. Fasting, exercise, and natural compounds have all been shown to trigger autophagy in various organisms. However, the safety and efficacy of these approaches may vary among individuals, and further research is needed to determine the optimal strategies for inducing autophagy in humans.
We will now discuss each of these methods more thoroughly, along with their potential benefits and considerations.
Fasting and Calorie Restriction
Fasting and calorie restriction have been shown to induce autophagy in various organisms, from yeast to mammals. By depriving cells of nutrients, fasting triggers a state of starvation, activating starvation induced autophagy to provide the energy required for survival. However, the optimal duration and intensity of fasting or calorie restriction for human autophagy remain unclear, and safety considerations must be taken into account, particularly for pregnant or breastfeeding individuals and those with certain medical conditions.
Despite these uncertainties, fasting and calorie restriction hold promise as potential strategies for modulating autophagy and promoting cellular health. Further research is needed to determine the optimal approach for each individual and to better understand the potential benefits and risks of fasting-induced autophagy.
Exercise and Physical Activity
Exercise has been found to trigger autophagy in response to cellular stress and damage, particularly in skeletal muscles. By activating pathways that promote autophagy, exercise may help cells maintain their health and function, contributing to overall well-being.
However, the specific mechanisms by which exercise induces autophagy and the potential benefits of this process require further investigation.
In the meantime, engaging in regular physical activity is a tried-and-true strategy for promoting overall health and well-being. By incorporating exercise into our daily routines, we may be able to support cellular health and autophagy, reaping the numerous benefits of this vital process.
Natural Compounds
Natural compounds, such as curcumin, have been shown to induce autophagy in animal models. Curcumin, a compound found in the spice turmeric, has been demonstrated to trigger autophagy in mice, and its potential therapeutic applications are being actively investigated.
However, more research is needed to determine the effects of curcumin and other natural compounds on human autophagy and their potential therapeutic applications, as suggested by Mizushima N.
The potential of natural compounds to modulate autophagy is an exciting area of research, with the possibility of discovering novel therapies that harness the power of autophagy for the treatment of various diseases. As our understanding of the molecular mechanisms underlying autophagy continues to grow, we may uncover new approaches for promoting cellular health and combating disease.
The Molecular Machinery of Autophagy
The molecular machinery of autophagy is a complex and intricate network of genes, proteins, and signaling pathways that work together to orchestrate the degradation and recycling of cellular components. This machinery is essential for the proper functioning of autophagy and plays a critical role in maintaining cellular homeostasis and preventing disease.
In this section, we aim to explore the key components of autophagy’s molecular machinery, such as autophagy-related genes (ATGs), signaling pathways, and selective cargo recognition systems.
Autophagy-Related Genes (ATGs)
Autophagy-related genes (ATGs) encode the proteins essential for the formation and function of autophagosomes, the double-membrane vesicles responsible for sequestering and degrading cellular components. Key players in this process include Atg5, Atg7, and LC3, which are involved in the formation and maturation of autophagosomes, as well as the recruitment and processing of cargo for degradation.
Understanding the functions and interactions of ATGs is vital for unraveling the molecular mechanisms of autophagy and developing targeted therapies for diseases associated with autophagy dysfunction.
Further research into the roles and regulation of ATGs will provide valuable insights into the complex and dynamic world of autophagy.
Signaling Pathways
Signaling pathways play a crucial role in the regulation of autophagy in response to nutrient availability and cellular stress. Two key signaling pathways involved in autophagy regulation, including the autophagy pathway, are mTOR and AMPK, which act as a master switch, turning autophagy on or off depending on the cellular environment.
mTOR is a serine/threonine kinase that is activated by growth factors and nutrients, promoting cell growth, proliferation, and survival by inhibiting autophagy. In contrast, AMPK is an energy-sensing enzyme that is activated by low energy levels, promoting energy metabolism and activating autophagy.
By understanding the complex interplay between these signaling pathways and their regulation of autophagy, we can gain a deeper appreciation for the intricate balance between cellular health and disease, as well as the role of intracellular pathogens in this process.
Selective Autophagy and Cargo Recognition
Selective autophagy is a process by which cells recognize and degrade specific cellular components, such as protein aggregates and damaged organelles, through the use of adaptor proteins like p62/SQSTM1. This selective recognition and degradation process allows cells to maintain their health and function by eliminating damaged or unnecessary components and recycling their building blocks.
Understanding the molecular mechanisms underlying selective autophagy and cargo recognition is essential for advancing our knowledge of autophagy and its role in health and disease. By identifying the key players and pathways involved in selective autophagy, we can develop targeted therapies that exploit the power of autophagy to combat disease and promote cellular health.
Monitoring and Measuring Autophagy
It is critical to monitor and measure autophagy for a clear comprehension of its role in health and disease, and to develop targeted therapies that can harness its potential. Various techniques have been developed for this purpose, ranging from the use of fluorescent markers and imaging to the application of biochemical assays.
These methods provide valuable insights into the dynamics of autophagy, its regulation, and its impact on cellular health and disease.
Fluorescent Markers and Imaging
Fluorescent markers, such as GFP-LC3, are powerful tools for visualizing autophagosomes and monitoring autophagy in living cells. By labeling specific autophagy-related proteins or structures and observing them through fluorescence microscopy, researchers can identify and quantify autophagic events, such as autophagosome formation and autophagic flux.
Employing fluorescent markers and imaging has considerably broadened our understanding of autophagy and its function in cellular health and disease. By providing real-time, dynamic information on autophagic processes, these techniques have opened up new avenues for investigating the molecular mechanisms of autophagy and developing targeted therapies that exploit its potential.
Biochemical Assays
Biochemical assays, such as Western Blotting and immunoprecipitation, are essential tools for measuring autophagy-related proteins and their interactions, providing insights into the molecular mechanisms of autophagy. These assays can be used to:
Quantify the levels of specific proteins
Determine their post-translational modifications
Investigate their interactions with other proteins or cellular components
By using these assays on mammalian cells, researchers can shed light on the complex interplay between autophagy and other cellular processes.
Applying biochemical assays in the study of autophagy has greatly enriched our understanding of this crucial process and its role in health and disease. By providing quantitative and qualitative information on the molecular machinery of autophagy, these assays have paved the way for the development of targeted therapies that harness the power of autophagy to promote cellular health and combat disease.
Summary
In conclusion, autophagy is a vital cellular recycling process that plays a crucial role in maintaining cellular health and preventing disease. The complex interplay between the molecular machinery of autophagy, its regulation by signaling pathways, and its role in health and disease highlights the importance of further research into this fascinating process. As our understanding of autophagy continues to grow, we may uncover new therapeutic strategies that harness the power of autophagy to promote health and combat disease, paving the way for a better understanding of the intricate balance between cellular health and disease.
Frequently Asked Questions
How long should you fast for autophagy?
Autophagy may start after 24-48 hours of fasting, though more research is needed to confirm ideal timing for humans.
Before making significant changes to your diet, it’s best to talk to a healthcare provider.
How do I know I’m in autophagy?
There are a number of signs to look for when determining if autophagy is taking place, such as elevated ketone levels, reduced appetite, improved cognitive function, reduced inflammation, temporary fatigue and less pain.
Furthermore, changes in hormones such as increased levels of glucagon can also indicate autophagy.
Why is autophagy bad?
Autophagy dysregulation can lead to abnormal cell growth and death, which in turn can cause disorders such as tumor formation.
Thus, autophagy can be detrimental in certain situations.
Is 16 hours fasting enough for autophagy?
Fasting for 16 hours has been proven to be an effective way to induce autophagy, with research reviews showing that it can be beneficial in aiding cancer treatments.
What are the three primary types of autophagy?
The three primary types of autophagy are macroautophagy, microautophagy, and chaperone-mediated autophagy, all of which play an important role in maintaining cell health.